-
How to Build an Atom – The Mechanics.
Posted on August 27th, 2015 No comments“So far I have been unable to find any property of matter that cannot be explained by the use of only electrons and nuclei.”
———————————————————
The construction of an atomic model that satisfies all the requirements of scientific knowledge regarding both physical and biological facts must be our starting point. It must be able to explain colour, weight (mass), state (i.e. solid, gas or liquid), changes due to temperature, changes due to pressure, hardness, and softness, rigidity and flexibility, chemical reactions, toxicity, radioactivity, gravity, magnetism, and most importantly, life.
My theory explains all known properties of matter. (It therefore passes the requirements of a theory.)
I may be wrong, but certainly not as wrong as the Physics Establishment.
The reader will have problems due to fact that I go against all the current atomic hypotheses that he/she will have been taught or accepted. I can understand this because I had the same problem myself, many times over the years, finding it difficult to believe my own results. Eventually, it was easier to consider existing hypotheses only to pinpoint where the problems were. This was a case of selecting any particular hypothesis proposed by the physics establishment, assume it is wrong, and work out alternative hypotheses and then produce a working theory. 90% of current hypotheses cannot satisfy the title of theory because there are no explanations of how they could work.
One of the main reasons for physicists opting for high-speed electrons is to try to explain the energy of an atom. However, all energy is in one of two types/states, momentum or stress. (Note; In rotary motion the energy is stored in a combination of momentum and stress.) All atoms are in a state of stress. The reader may find this statement difficult to accept but it is true, whether you are considering chemistry, radiation or mechanics.
Fig. 1 below shows the image of the atom best known by the public.
Fig. 2
 Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.
Fig. 2 shows the current hypothetical atom ‘structure’, which is a slightly less haphazard version of Fig. 1.Neither of these models is of any use in explaining the known properties of matter. How do two or more atoms form a molecule without all the electrons colliding? How can one atom be harder than another? How is one atom a liquid and another a solid?
Ask your local physicist any of these questions and he will mumble that it is too complicated to explain to mere mortals. The fact is that modern physics cannot explain any of them.
If electrons are not whizzing (whizzing is travelling at high speed) around causing dangerous collisions then it is easy to form molecules. All you have to do is to remove one electron from one atom and fill the gap by moving another atom so that one of its electrons fits in the gap in the first atom. Dead easy, you now have a molecule, two atoms jointed by a mutual electron. Both atoms have the right number of electrons therefore nothing has changed but we now have a molecule. However, life, physics and mechanics are not that simple.
Modern physics argues that the more electrons there are in an atom then the heavier it is. Therefore, as our molecule is an electron short of two atoms, it should be lighter than the original two atoms. Unfortunately, I totally disagree with this hypothesis because it is illogical.
The Structure of Atoms and the origin of Mass.
If Hydrogen is the lightest element and only has one atom, the one to one bond between the nucleus and the electron would make it far more difficult to remove this one electron from Hydrogen than trying to remove one electron from an atom with many electrons. (See Proton.) However, Hydrogen is the easiest element from which to obtain energy in the form of emitted electrons. In addition, Hydrogen has a ‘soft’ explosion indicating that the velocity of emitted electrons is relatively low. Although there are a few arguments (but not facts) against this, none of them overcomes the other problems of an atom with orbiting electrons.
If we reverse the hypothesis and say that the greater the number of electrons the lighter the atom, then this explains away many of the problems in physics without introducing any more. This would mean that the heaviest possible atom would have one electron.
It would also mean that the lightest detectable element, Hydrogen, would have the second largest number of electrons.
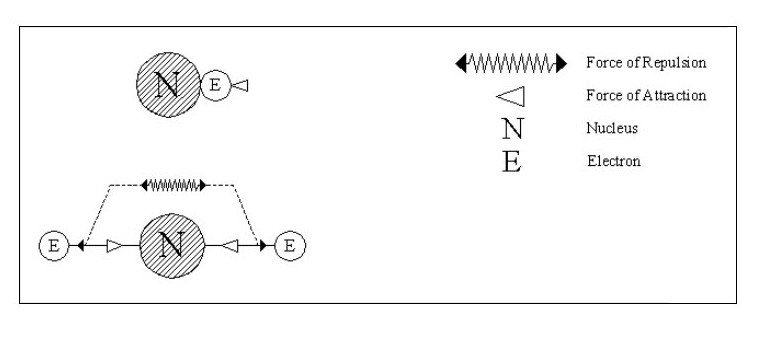
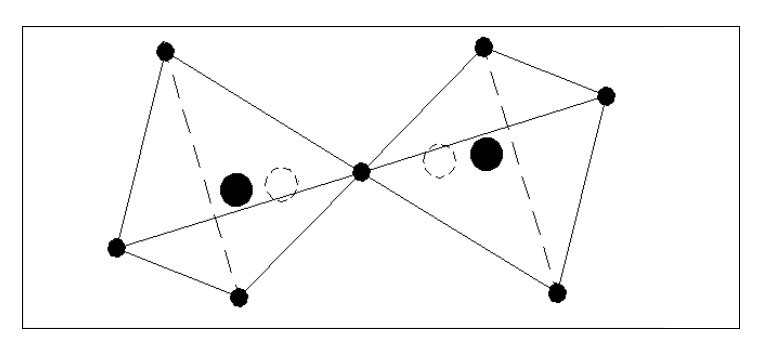
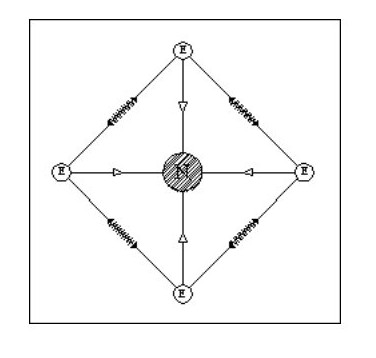
Let us consider some simple possible atoms. In Fig. 3, the top sketch shows a nucleus with a single electron. As this electron is not whizzing around it snuggles up against the nucleus due to the force of attraction. The lower sketch shows an atom with two electrons. In this case, the force of repulsion between the two electrons is acting against the force of attraction between the electrons and the nucleus. The electrons would align themselves on opposite sides of the nucleus. Although I have shown the electrons standing well clear of the nucleus, they could still be pressing against the nucleus but with less force. This would depend on the relative strengths of the attraction and repulsion forces.
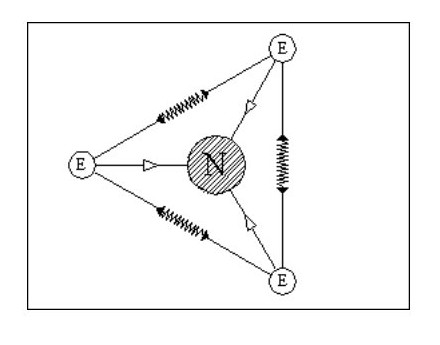
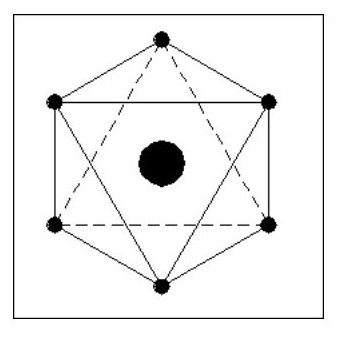
Let us add another electron. In Fig. 4, the three electrons have arranged themselves around the nucleus so that the forces of attraction and repulsion are balanced.
Now let me explain the problem that is irritating you. How can we have flat atoms? If the atom was a mile away from the nearest other atom or available electrons then Fig. 4 would be its natural state. In close proximity to other atoms, it would tend to combine with these atoms to form complex molecules, or try to accumulate more electrons.
It should be remembered that Fig. 4 is not a Lithium atom, it is an atom that is extremely heavy and one, like our previous two atoms, only being found at the core of collapsed stars or far out in space.
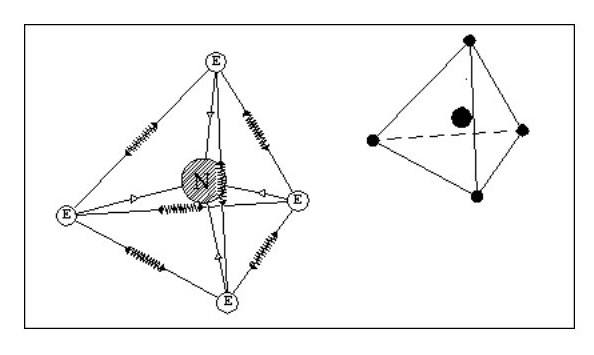
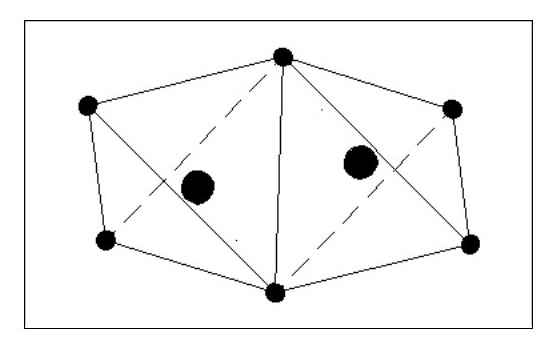
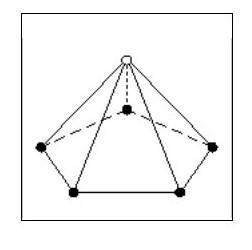
Let us now consider an atom with four electrons. Fig. 5 shows the atom in its ‘natural’ state.
 However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6
However if in close proximity to other atoms it would tend to form a tetrahedron as shown in Fig.6Now that we have arrived at our first three-dimensional atom let us consider molecules. Using Fig. 6, we can create a simple molecule by using a single common electron.
With this type of molecular bond, it is obvious that the molecule will be quite flexible because one atom can flex and rotate relative to the other.
We now come to another deviation from modern physics. It is considered that for electrons to be attracted to the nucleus, the nucleus must have a positive charge (See Proton). Looking at the evidence from most experimentation would indicate that:
A. The nucleus is neutral and that the electron is attracted to the neutral state. Or
B The nucleus is attractive to both electrons and other nuclei, but the surrounding electrons reduce the affinity for other nuclei.
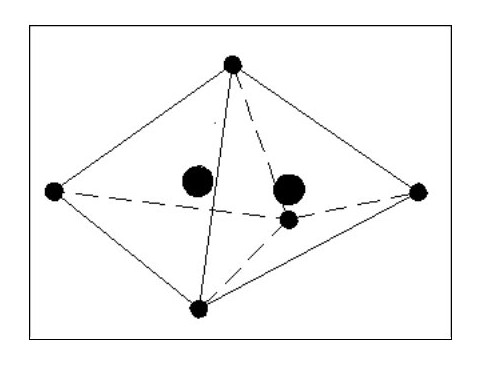
I feel that B. is the most logical. In the above sketch (Fig. 7) the shared electron presents a weakness in the surrounding shield of electrons, and the mutual attraction between the nuclei would pull them closer together as shown by the dotted circles. This in turn would distort the shapes of the two atoms as the forces balance. This weakness would be present in all molecules with similar results.
Let us now consider that these two atoms are joined as a molecule but by two common electrons instead of one. The formation of this molecule releases two electrons, therefore making the molecule heavier than the previous molecule. This new molecule is also less flexible than the previous one, one atom effectively being hinged off the other.
It should be considered that we are still dealing with extremely heavy atoms at this stage and the removal of electrons requires great pressure or a high energy.
If we now take our two atoms and create a molecule using 3 common electrons ( See Fig. 9 Below), we lose most of the flexibility and have an even heavier (and harder) molecule. It is clear that from two identical atoms we can produce the option of 3 different molecules each having different properties.
Now consider this point, if we split this molecule we are 3 electrons short of our original two atoms.
We could end up with one of our original atoms plus an atom with a single electron.
The modern physicist would argue that this result proves that the molecule was created by the addition of two totally different atoms. If it was difficult or impossible to split the molecule then the modern physicist would argue that this proves that the molecule is actually an atom!
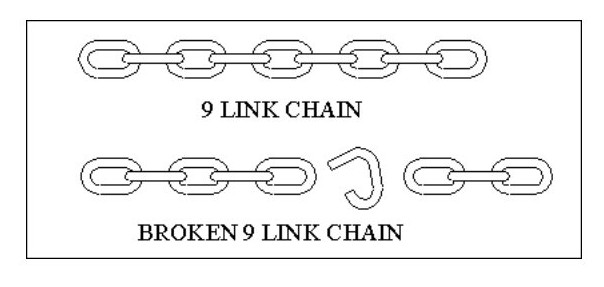
Most of our known substances considered to be atoms may in fact be difficult to split molecules, and many of our molecules may be conglomerations of identical atoms, but which when separated produce two or more totally different atoms. In the sketch below if the chain breaks as shown you could mistakenly argue that the 9 link chain is obviously created from a 5 link chain and a 3 link chain.
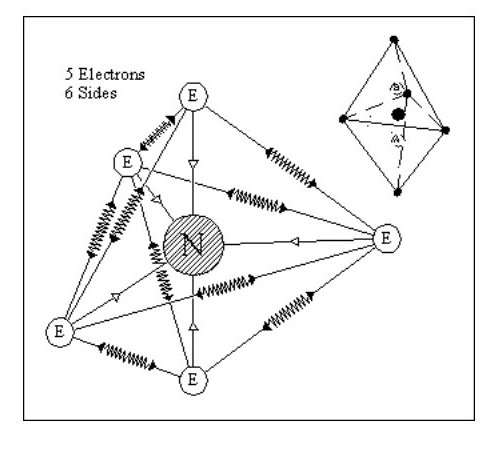
Let us now consider an atom with 5 electrons. We have a problem with this in that it is difficult to balance all the forces. On the other hand it gives us a glimmering of the reason for the life force.
Although similar in shape to Fig. 9 it only has one nucleus which can move between A and B with little effort, seriously distorting the shape of the atom in the process. This distortion would not destroy the atom, the overall forces being strong enough to retain its integrity.
It would take little effort to set up an oscillation in this atom. If an oscillation was set up and the atom shielded from all external forces, the oscillation could continue forever.
See Atomic Damping.
We are still looking at very heavy atoms, the weight/mass detectable being dependent on the number of electrons. The actual mass of an atom remains unchanged as this is the mass of the nucleus, which does not change. The electrons mask the affect of the nucleus’ mass. With a full complement of electrons the atom will have no detectable mass.
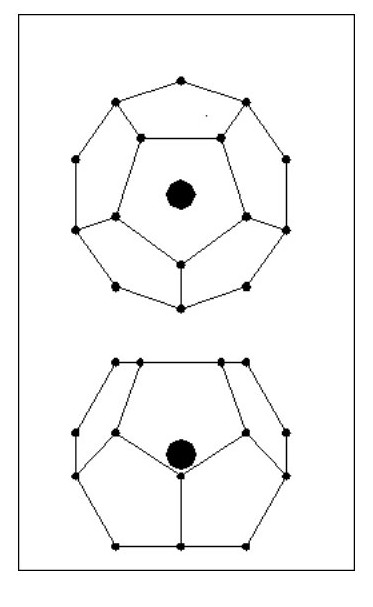
We will now look at a lighter atom. We will consider an atom in the shape of a Dodecahedron i.e. 12 sides and 20 electrons as Fig. 14.
(You might also like to look at The Origin of the Golden Section., because ratios relating to the dodecahedron tie in with golden section ratios.)
All the electrons lie on a sphere, equally spaced and all the same distance from the nucleus. Obviously 20 electrons will not mask the affect of the nucleus’ mass. [Note: With regard to the dodecahedron shape, this would clearly allow for molecular bonding with 1, 2 or 5 common electrons. However, under certain conditions 3 and 4 electron bonds could occur.]
Can we increase the numbers of electrons without causing problems?
If we add 12 electrons they will be attracted to the nucleus but will have problems with the repulsion forces of the existing electrons. These repulsion forces are weakest in the centres of the faces so the new electrons will align themselves in the centre of the faces.
But consider the following point: The existing electron sphere reduces the attraction forces for the new electrons and each new electron has to contend with the repulsion forces of 5 existing electrons. Our original dodecahedron will now have a five sided spike on each face as Fig. 15.
 It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.
It is clear that the new electrons will not be on the same sphere diameter as the existing ones, they will form a second sphere. We now have an inner shell with 20 electrons and an outer shell with 12 electrons.The new electrons will be equally spaced around the outer shell and will all be the same distance from the nucleus.
Now if we can have 12 electrons in a shell outside the dodecahedron, can we have a shell inside it containing 12 equally spaced electrons? The answer is yes. We can also have another shell on the outside containing 20 electrons, followed by further shells having alternatively 12 and 20 electrons.
The limit will be reached when the number of electrons completely mask the attraction force of the nucleus.
Interim Recap.
The orbiting electron hypothesis does not explain any of the properties of matter. See note.
- My theory explains rigidity and resilience.
- My theory so far gives a faint insight into the possible cause of the life force.
- My theory gives a logical cause for the crystal structure of many materials.
- My theory explains molecular bonding.
- My theory explains weight/mass. See note.
Note: The existing hypothesis states that the number of electrons governs the mass/weight of the atom. It does not explain how. The original reason for the statement that hydrogen had one electron was not based on any scientific reasoning or facts.
My readers may be wondering when I am going to explain about quarks, pi-mesons, etc. etc.
The answer is that there would be no point in wasting valuable space. The majority of these are hypothetical particles dreamed up to try and balance the faults in the existing weak hypotheses. Note; So far I have been unable to discover any property of matter that cannot be explained using only electrons and nuclei.
Let now consider an atom with six electrons. This would be in the form of 8 triangular faces.
As with the dodecahedron we could add an extra shell of electrons, in this case 8 of them. These electrons would would form a slightly distorted cube. If we add further shells they each would have 8 electrons until the forces blanketed the nucleus.
Now to answer your question as to how many electrons does it take to blanket the nucleus. I just don’t know. The lightest known substance is hydrogen. No-one knows how many electrons each atom of hydrogen has despite the physicists statement that it has only one.
The problem is that we really don’t know how many electrons any atom has. None of the experiments in the last 100 years has produced any logical figures and the standard tables of the elements can only be considered as pure guesswork.
We will take an arbitrary figure of 100 as the number of electrons required to blanket the nucleus.
Logically it would only take one additional electron to finally trip an atom into a condition that would make it undetectable. In the above atom the electrons are added in batches of 8. In the dodecahedron shaped atom the electrons are added in batches of 12 or 20. Obviously both families of atoms could get to the stage of being undetectable. It would appear to be unlikely that in either case would the final shell would need a full complement of electrons before the atom became undetectable.
However it just may require a full complement or at least a balanced part complement.
The Structure and Mechanics of Atoms and Mousetrap molecules
Let us consider chemistry and chemical reactions. Chemistry relates to the formation of molecules.
If you put powdered copper, zinc, carbon, titanium plus water into a tray and mix them, once they have settled you will still have powdered copper, zinc, carbon, titanium plus water in the tray. No new molecules will have been created. Why not?
Cu + C + Zn + Ti + H20 = ?????
You cannot get an answer for the above equation because it does not allow for the two most important variables; Temperature and pressure. (I’ve never come across a valid formula for a cup of tea either.)
Without variables no formulae are valid.
Under ‘natural’ conditions certain molecules can be created. Obviously ‘natural’ conditions vary depending on where you are. Natural conditions at the North Pole are different than natural conditions in the Sahara Desert. ‘Natural’ conditions on the Moon are different conditions than on Pluto or Mercury. Under ‘natural’ conditions many molecules cannot be created i.e. they need high /low pressure or high/low temperature or a combination.
Under ‘non-natural’ conditions many molecules can be created.
Most life on Earth evolved to suit gradually changing ‘natural’ conditions. Occasionally, unnatural conditions, such as volcanic activity occur that release molecules that have been created under unnatural conditions such as high temperatures and high pressures. Many of these molecules are toxic to most forms of life on Earth. Some lifeforms, such as the sea creatures around volcanic vents on the ocean floors, have evolved to handle the toxins, high pressure and high temperatures. To these creatures these conditions are now ‘natural’.
Under higher pressures atoms contract and under lower pressures atoms expand. If the pressure is high enough electrons are ejected from atoms (Exothermic reaction). THEREFORE they become different atoms that have qualities different from the original atoms.
Lighter atoms compress more than heavy atoms, therefore they will lose electrons easier than heavy atoms.
The gas Nitrogen has a completely different atom structure than liquid nitrogen.
Carbon and diamond have different atomic structures.
Water and Ice have different atomic structures.
If you place two dissimilar atoms that, (under normal conditions would not form a molecule), under increasing pressure you will arrive at condition such that the lighter atom would be able to form multiple electron bonds (MEBs) with the heavier one.
When this happens the molecule would be stable at this particular pressure. However, if the pressure is released, the molecule will either split into two separate atoms again or will remain as a molecule but with the lighter atom being in a distorted and stressed condition. This would make it vulnerable to forming MEBs with other atoms that it would not be possible under normal conditions.
Mousetrap Molecules
In affect, the molecule could become a ‘mousetrap molecule’ that could be easily triggered into forming strange/unnatural molecules or atoms, or even molecular explosions. Apart from toxins this would explain ‘radio activity’ and inflammability.
Radioactivity depends on a source of materials that have been subject to high pressures. Most of these are relatively stable in that only a few molecules per/second explode in any given mass. However, when they do the lighter atom disintegrates at high speed. Impurities in the ore tend to restrict the amount of radioactivity. Purifying the ore increases the ratio of mousetrap molecules until they start to trigger each other and you get a sustained reaction
————————————–
NOTE; Free Electrons have a very important role in chemistry. Free electrons are those not locked into the structure of an atom. They may be attracted to nearby atoms but can be easily moved around without affecting the structure of the atoms. Free electrons will still have a blanketing/insulating effect on the nucleus of the atom that would hide its true mass.
(Structural Electrons are those electrons that are part of the structure of atoms.)
Heat and Temperature.
Let me make the following statement: ATOMS DO NOT HAVE A TEMPERATURE.
Any isolated atom is completely devoid of any sense of temperature, it is neither hot, warm or cold.
If it loses an electron then this electron MAY be detected as heat.
Under pressure, atoms will reach a stage when the atomic structure starts to break down and electrons are emitted.
Temperature measurement in this case is dependent on its emission velocity.
——————————————————-
The mass of an atom is a force.
Let me apologise for the title of this post. You cannot build an atom. You can only disassemble an atom.
I was going to change the title but could not think of a better one.
———————————————-
Families of Atoms
MORE TO COME SHORTLY
Author; Brian Williams
Comments accepted.
-
Individuals names only, no company names.
-
Only one URL allowed.
-
Note that comments are now processed through a
separate computer.
Comments automatically deleted. (I do not see them.)
-
All comments from pornographic sites.
-
All comments from sales sites.
-
All comments not relevant to my website.